| Barium Tungstate Nanoparticles | |
| Product No | NRE-5023 |
| CAS No. | 7787-42-0 |
| Formula | BaWO4 |
| APS | <100nm (Can be Customized) |
| Purity | 99.9% |
| Color | Off-White |
| Molecular Weight | 385.16g/mol |
| Density | 5.04g/ml |
| Melting Point | 5.04 g·cm−3 (25 °C) |
| Boiling Point | NA |
Barium Tungstate Nanoparticles
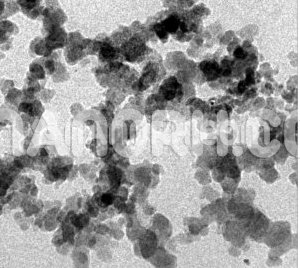
Barium Tungstate is an inorganic compound made of barium and tungsten. It exists in the form of a white crystalline powder and is widely recognized for its high density, high atomic number, and excellent stability. In its nanoparticle form, barium tungstate exhibits enhanced properties compared to its bulk counterpart, such as a high surface area, increased reactivity, and improved optical and electronic characteristics. The particle size of barium tungstate nanoparticles typically ranges from 1 to 100 nanometers, giving them unique behaviors at the nanoscale.
Barium tungstate nanoparticles have become a subject of increasing interest due to their applications in various fields, including photonics, medicine, radiation detection, and material science. The unique properties that these nanoparticles exhibit at the nanoscale enable them to be employed in innovative technologies, including catalysis, energy storage, medical imaging, and luminescent devices.
Features
High Density and Atomic Number:
Barium tungstate has a relatively high density (approximately 6.17 g/cm³), and a high atomic number for both barium and tungsten. This makes it an ideal material for radiation shielding and detection applications. These features are particularly useful in medical imaging (such as X-rays and CT scans) and gamma-ray detection.
Luminescent Properties:
Barium tungstate nanoparticles exhibit strong luminescent properties. They have the ability to emit light when exposed to external energy sources (such as ultraviolet or X-ray radiation). The photoluminescence and radioluminescence properties make barium tungstate nanoparticles highly suitable for optical applications, including phosphors, light-emitting devices (LEDs), and displays.
High Stability:
One of the key features of is their chemical stability. They are resistant to corrosion, oxidation, and thermal degradation, making them durable and long-lasting in a variety of harsh environments. This stability is particularly important for high-performance applications in electronics and radiation detection.