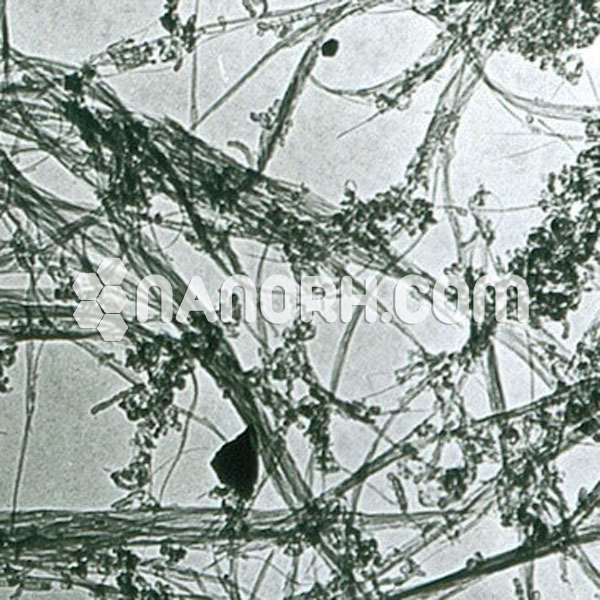
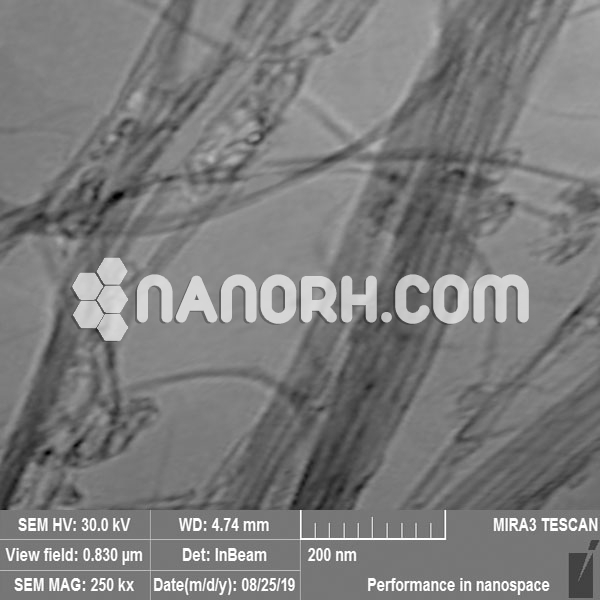
| COOH Functionalized Short MWCNTs | |
| Product No | NRE-35002 |
| CAS No. | NA |
| Purity | Carbon nanotubes > 95wt% |
| Average Diameter | 5-15 nm |
| Average Length | 0.5-2 um (TEM) |
| Special Surface Area(SSA) | >233m2/g(BET) |
| Tap Density | 0.15g/cm3 |
| True Density | 2.1g/cm3 |
| Electric Conductivity | > 100 S/cm |
COOH Functionalized Short MWCNTs
Carbon nanotubes (CNTs) are cylindrical nanostructures made from carbon atoms, and they are widely known for their extraordinary mechanical, electrical, and thermal properties. CNTs are generally categorized into two types: single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs). MWCNTs are composed of several concentric graphene sheets rolled into a tube-like structure, and they exhibit enhanced mechanical strength, electrical conductivity, and chemical stability compared to single-walled CNTs. These properties make MWCNTs highly attractive for a wide variety of applications in fields such as electronics, materials science, energy storage, and biomedical engineering.
However, the widespread use of MWCNTs is often hindered by issues such as their aggregation tendency, poor dispersion in solvents, and limited compatibility with many materials. To enhance the performance and expand the applications of MWCNTs, functionalization techniques are employed to modify the surface of these nanotubes. One of the most common methods of functionalizing MWCNTs is the introduction of carboxyl groups (-COOH) onto the surface, which improves their solubility, dispersion, and reactivity.
COOH Functionalization of MWCNTs
Functionalization refers to the chemical modification of a material’s surface to alter its properties. In the case of MWCNTs, functionalization with carboxyl groups involves introducing -COOH groups onto the sidewalls or ends of the nanotubes, typically through oxidation methods. This process enhances the surface characteristics of the nanotubes, making them more compatible with various solvents, matrices, and other materials.
The functionalization process typically begins with the oxidation of the MWCNTs using strong acids such as nitric acid (HNO₃) or a mixture of sulfuric and nitric acids. These acidic treatments generate oxygen-containing functional groups, such as carboxyl (-COOH) and hydroxyl (-OH) groups, on the surface of the nanotubes.