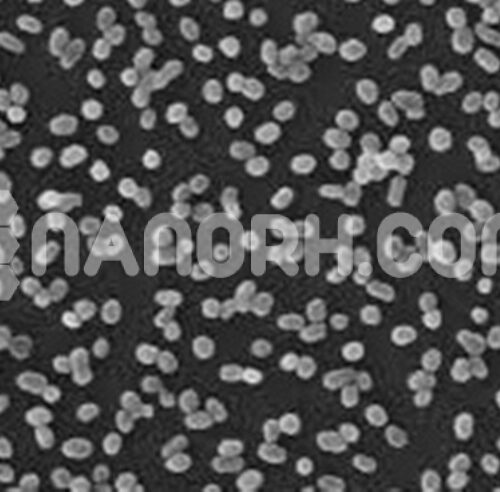
| Copper Selenide Powder | |
| Product No | NRE-11080 |
| CAS | 20405-64-5 |
| Purity | 99.9% |
| Formula | Cu2Se |
| APS | <40 µm (Can be Customized) |
| Color | Dark blue |
| Molecular Weight | 206.05 g/mol |
| Density | 6.84 g/cm3 |
| Melting Point | NA |
| Boiling Point | NA |
Copper Selenide Powder
Copper selenide is a chemical compound composed of copper and selenium. It has a variety of applications across different fields due to its unique properties. Copper selenide powder finds uses in various industries and research fields, some of which include:
Photovoltaic Devices: Copper selenide, due to its semiconducting properties, is used in the production of thin-film solar cells. These cells are used to convert solar energy into electrical energy. It has been studied for its potential application in the development of low-cost and efficient solar cells.
Thermoelectric Materials: Copper selenide is also investigated for its potential in thermoelectric applications. These applications involve utilizing temperature differences to generate electricity. Copper selenide-based materials can potentially be used in thermoelectric devices for waste heat recovery and other related applications.
Catalysis: Copper selenide powder is used as a catalyst in various chemical reactions, including organic transformations and industrial processes. Its application as a catalyst is based on its ability to facilitate chemical reactions without being consumed in the process.
Semiconductor Industry: Copper selenide finds applications in the semiconductor industry for the development of various electronic components. It can be used in the production of diodes, transistors, and other electronic devices.
Research and Development: Copper selenide powder is extensively used in research and development activities, particularly in the fields of material science and solid-state physics. Researchers study its properties and behavior under different conditions to discover new applications and improve existing technologies.
Chemical Synthesis: Copper selenide is also used as a precursor in the synthesis of other compounds and materials. It serves as a building block for the preparation of more complex chemical structures.
Optoelectronic Devices: Due to its semiconducting properties, copper selenide is utilized in the production of various optoelectronic devices, such as light-emitting diodes (LEDs) and photodetectors.
Thermal Imaging: Copper selenide is also used in some advanced thermal imaging technologies, where its properties enable the detection and visualization of heat differences in various applications, including military and industrial fields.