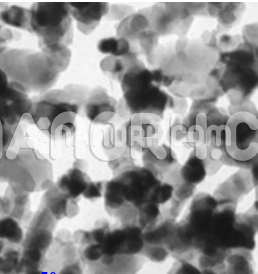
| Lead Sulfide Nanoparticles | |
| Product No | NRE-5129 |
| CAS No. | 1314-87-0 |
| Formula | PbS |
| APS | <100nm (Can be Customized) |
| Purity | 99.9% |
| Color | Black |
| Molecular Weight | 239.30 g/mol |
| Density | 7.60 g/cm3 |
| Melting Point | 1118 °C |
| Boiling Point | 1281 °C |
Lead Sulfide Nanoparticles
Introduction
Lead sulfide nanoparticles is an inorganic semiconductor material composed of lead (Pb²⁺) and sulfur (S²⁻). It has a narrow band gap of approximately 0.37 eV at room temperature, which makes it an excellent material for infrared (IR) applications and optical devices. PbS is commonly found in nature as the mineral galena, but in nanoparticle form, it exhibits significantly altered properties due to quantum confinement effects and increased surface-to-volume ratios..
Properties
Quantum Confinement Effects:
Due to their small size (typically a few nanometers), PbS nanoparticles exhibit quantum confinement effects that alter their electronic and optical properties compared to bulk PbS. These effects result in tunable optical absorption and emission spectra. The band gap of PbS nanoparticles can be controlled by adjusting their size, making them suitable for a wide range of photonic applications.
Narrow Band Gap:
PbS has a narrow direct band gap, which allows it to absorb light in the infrared (IR) region, making it a promising material for infrared photodetectors, thermal imaging, and IR light harvesting.
Strong Photoluminescence:
PbS nanoparticles exhibit strong photoluminescence (PL) properties, which can be further enhanced by controlling the particle size. This makes them useful in fluorescence-based applications, such as biological imaging and optical sensing.
High Charge Carrier Mobility:
As a semiconductor material, PbS nanoparticles demonstrate good charge carrier mobility, which is essential for applications like photovoltaics, photoelectronics, and energy storage devices.
Enhanced Surface Reactivity:
Due to their high surface-to-volume ratio, PbS nanoparticles have a high surface reactivity, which can be exploited for catalysis, sensor applications, and adsorption processes.