|
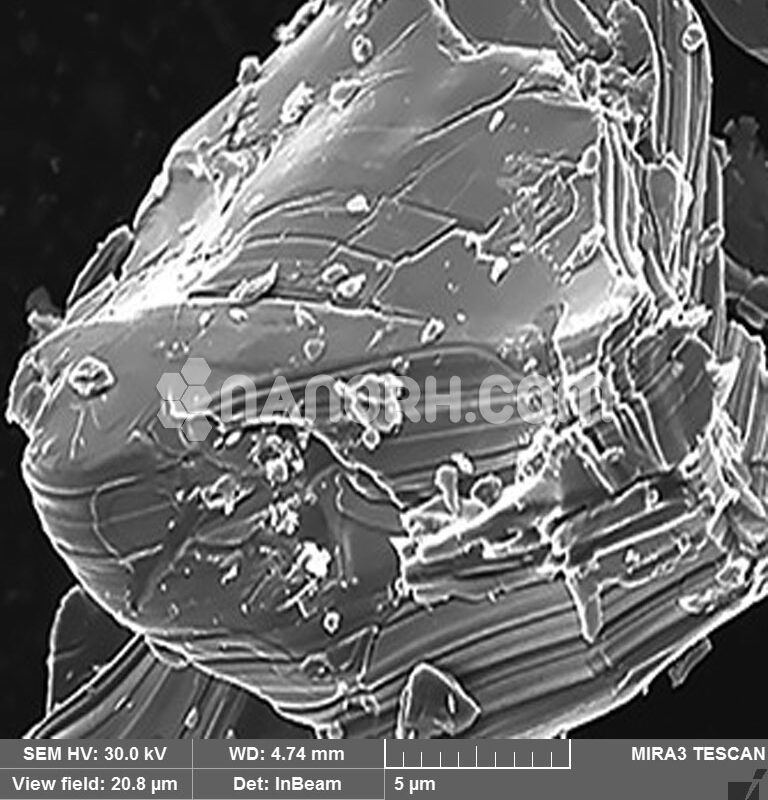
Niobium Carbide MXene Powder |
|
| Product No. | NRE-59002 |
| CAS No. | 12011-99-3 |
| Formula | Nb4C3 |
| Molecular Weight | 407.65 g/mol |
| APS | < 40 μm |
| Purity | 99.9% |
| Form | Powder |
Niobium Carbide MXene Powder
Niobium carbide (NbC) is a type of MXene, which is a class of two-dimensional (2D) materials derived from transition metal carbides, nitrides, or carbonitrides. MXenes are known for their unique combination of metallic conductivity, hydrophilicity, and high surface area, making them promising for various applications in energy storage, catalysis, and electronic devices.
Niobium Carbide MXene Powder:
- Structure and Composition: Niobium Carbide Mxene Powder has a general formula of Nb2C. It is derived from the selective etching of aluminum from a parent MAX phase (where “MAX” refers to a family of materials with the formula M_n+1AX_n, where M is a transition metal, A is an element from groups 13-14, and X is carbon or nitrogen).
- Synthesis: The synthesis of NbC MXenes typically involves the following steps:
Preparation of the MAX phase: The precursor MAX phase, such as Nb2AlC, is synthesized through methods like solid-state reaction.
Selective etching: Aluminum is selectively etched from the MAX phase using acidic solutions, such as hydrochloric acid (HCl) with fluoride salts, resulting in the formation of NbC MXene.
Post-processing: The obtained NbC MXene is often washed and exfoliated to obtain a stable powder form.
- Properties: NbC MXenes exhibit excellent electrical conductivity, making them suitable for applications in electrodes for energy storage devices. Mechanical Strength: They have good mechanical strength and stability. Chemical Stability: NbC MXenes are relatively stable in various chemical environments, contributing to their versatility in different applications.
Applications
- Energy Storage: Due to their high electrical conductivity and large surface area, NbC MXenes are used in supercapacitors and batteries.
- Catalysis: They can act as catalysts or catalyst supports in various chemical reactions.
Sensors: Their high surface area and conductivity make them useful in the development of sensitive and selective sensors.
The field could lead to enhanced performance in energy storage devices, more efficient catalysts, and advanced electronic materials.