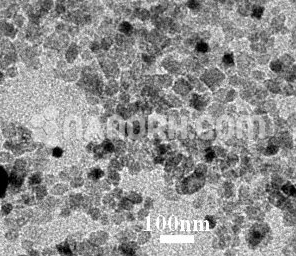
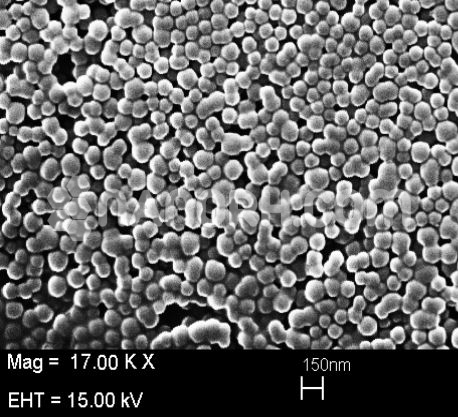
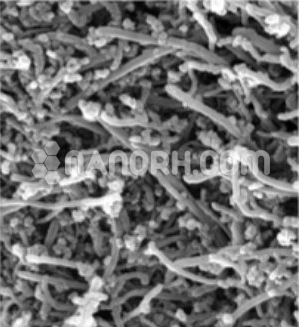
Precipitated Calcium Carbonate Nanoparticles / Precipitated CaCO3 Nanopowder, 50nm, 40wt%, Water Dispersion
Above 710 oC, melting bismuth oxide can erode or dissolve metal oxides. The main purpose: the electronics industry; pressure-sensitive resistors; Capacitance of major doping materials; medicine; artificial bone imaging; glass; Bismuth oxide in ceramics can increase the index of refraction of glass
| Precipitated Calcium Carbonate Nanoparticles Water Dispersion | |
| Product No | NRE-23043 |
| CAS No. | 471-34-1 |
| Formula | CaCO3 |
| Molecular Weight | 100.0869 g/mol |
| APS | <50nm (can be customized) |
| Purity | 98% |
| Density | 2.71 g/cm³ |
| Color | White |
| pH | 8-10 |
| Solvent | Water (as per requirement) |
Precipitated Calcium Carbonate Nanoparticles Dispersion
Precipitated Calcium Carbonate Nanoparticles Despersion
Precipitated Calcium Carbonate nanoparticles Dispersion are derived from carbonate rocks. Carbonates are made of particles. Most carbonate rocks derive from the accumulation of bio clasts created by calcareous organisms. Carbonate rocks usually form in areas that favor biological activity, that is, in shallow and warm seas, in areas with little or no siliciclastic contribution. Meta carbonates are metamorphosed limestone rocks in which the carbonate component is predominant, with a grain blastic polygonal texture. Carbonate and meta carbonates are the most widely used raw materials in construction applications with a recorded history. Carbonate and meta carbonate rocks are usually converted into lime after calcination, as quicklime (CaO) and slaked lime Ca (OH) 2. Both forms have different industrial uses, i.e. to neutralize acid waste, as fillers in pulp and in paper industry and as a flow in the steel industry, as well as uses in road construction, gold recovery and other environmental applications. Carbonate and meta carbonate rocks usually occur as limestone, marble, travertine, gypsum, coquina, tuff, stalactites and stalagmites in karst regions. The primary sedimentary carbonate rocks for the formation of marble are more commonly limestone or dolostone composed of recrystallized carbonate minerals. Precipitated Calcium Carbonate Nanoparticles Despersion is found in several crystalline polymorphs at ambient pressure. Among them, calcite is known to be the most stable phase in ambient atmospheric conditions. The formation of any of these three polymorphs strictly depends on some parameters such as the temperature, supersaturation and pH of the reaction solution. Carbonate rocks have been used in the cement industries and construction engineering for several years in the form of ground Calcium Carbonate. PCC is purer than the carbonate rock from which it is made and is poorer in silica, magnesium and lead. The morphology and size of PCC are different from that of GCC. GCC is seen to have an irregularly rhombohedral shape at high magnification. The morphology of the PCC crystal depends on the final product and the particles are mostly uniform and regular compared to the GCC.