|
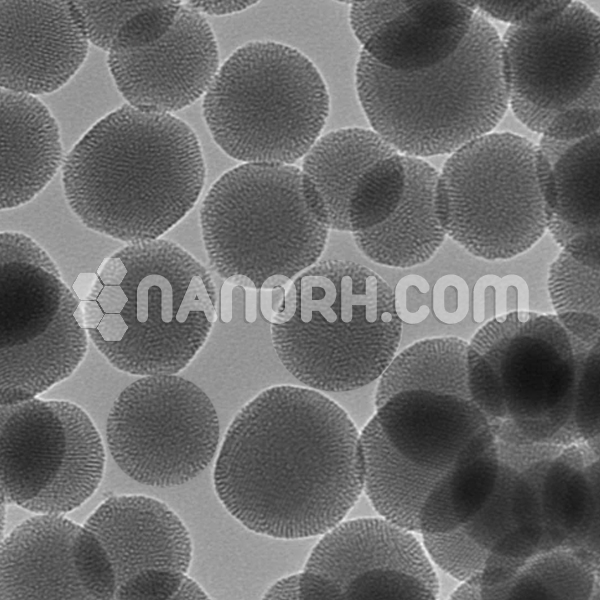
Silica Mesoporous SBA-16 |
|
| Product No. | NRE-590110 |
| CAS No. | 7631-86-9 |
| Linear Formula | SiO2 |
| SSA | 500 – 700 m2/g |
| Pore Diameter | 4 – 6 nm |
| Purity | 99.9% |
| Form | Powder |
Silica Mesoporous SBA-16
Silica Mesoporous is inorganic material that is synthesized in the presence of surfactants that form polycondensation of silica species, which come from different sources of silica. Synthesis conditions such as: source of silica, type of surfactant, ionic strength, pH and composition of the reaction mixture, temperature and duration of synthesis affect the micellar conformation of the surfactant, the interaction between silica and surfactant and the degree of polycondensation of the Silica Mesoporous. These conditions determine the properties of the porous structure (type of mesostructure, diameter and volume of the pores, wall thickness) and the macroscopic morphology. A variety of ionic and nonionic surfactants have been used to obtain materials with different porous and morphological properties. MCM-41, MCM48 and MCM-50 mesoporous silica (MCM: Mobile Crystalline Material) with hexagonal, cubic and lamellar mesostructures and different morphologies were synthesized using alkylammonium surfactants and TEOS or sodium silicate in a base medium. Their pore size and wall thickness do not exceed 4.0 nm and 2.0 nm, respectively. Using cationic surfactants in acidic environments, the first SBA-type materials with different mesostructures were obtained, with porous properties similar to MCM-X materials. SBA-15 and SBA-16 silicon dioxide (SBA: University of Santa Barbara) with larger pore sizes and thicker walls are produced using nonionic surfactants derived from poly (propylene oxide) and poly (ethylene oxide) in an acidic environment. Other materials called MSU-X (Michigan State University) are synthesized with nonionic surfactants at neutral pH, but their structures are more disorganized and the pore diameter and wall thickness are approximately 2.1-8.0 nm and 1.5-4.0 nm. . By replacing TEOS with sodium silicate in the presence of nonionic surfactant, it is also possible to obtain silica with a controlled morphology. The pore size can be modulated during synthesis by controlling the reaction time and temperature, using swelling organic molecules such as aromatic hydrocarbons and trialkylamines, regulating the concentration of surfactant and cation or by post-synthesis treatment such as water treatment, amine or by changing the liming conditions. The control of morphology by the synthesis conditions was explained by the influence of defects during the nucleation and growth process or by the formation mechanism.