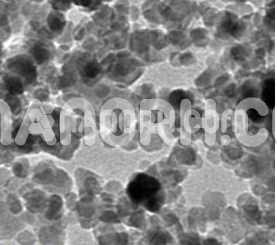
Cu-Ni Alloy Nanopowder/Nanoparticles (99.9%, <100nm)
Damp reunion will affect its dispersion performance and using effects, therefore, this product should be sealed in vacuum and stored in cool and dry room and it should not be exposure to air. In addition, the product should be avoided under stress.
| Cu-Ni Alloy Nanoparticles | |
| Product No | NRE- 2012 |
| CAS No. | 7440-50-8/7440-02-0 |
| Formula | Cu-Ni |
| APS | <100nm (Can be Customized) |
| Purity | 99.9% |
| Color | Grey-black |
| Molecular Weight | 122.2394 g/mol |
| Density | 8.96 g/cm3 |
| Melting Point | 1145°C |
| Boiling Point | NA |
Cu-Ni Alloy Nanopowder
Cu-Ni alloys are copper alloys (base metal with the highest individual content) and nickel with or without other elements, so the zinc content cannot exceed 1%. When other elements are present, nickel has the highest individual content after copper, compared to any other element.
As with other copper alloys, it is necessary to distinguish between forged alloys, which are processed to obtain semi-finished products, and melting alloys, from which the jets are produced by various melting processes.
In addition to 8.5-45% Ni, most commercial alloys generally contain manganese, iron, and tin to improve their specific properties, the molten alloys also contain niobium and silicon additions.
The hardened copper, nickel and silicon alloys with an age between 1.0 and 4.5% Ni and 0.2 to 0.6% of Be are not discussed here. In European standards, these alloys are assigned to “alloys of low alloys copper” (see CR 13388 and relevant product standards).
In 1751, A.F. Cronstedt managed to isolate the nickel. However, Cu-Ni alloys existed much earlier, mostly prepared by processing minerals. Nowadays, Cu-Ni alloys have acquired a variety of interesting applications due to their specific characteristics.
Copper and nickel are adjacent to each other in the periodic system of the elements, with atomic numbers 29 and 28 and atomic weights of 63.54 and 68.71. The two elements are closely related and completely miscible both in the liquid state and in the solid state. Cu-Ni alloys crystallize across the concentration range in a face-centered cubic network. The separation of the network from the solid center-to-face cubic solution varies almost linearly with the atomic concentration between the values of copper (3,6153,10-8 cm) and of nickel (3,5238,10-8 cm).