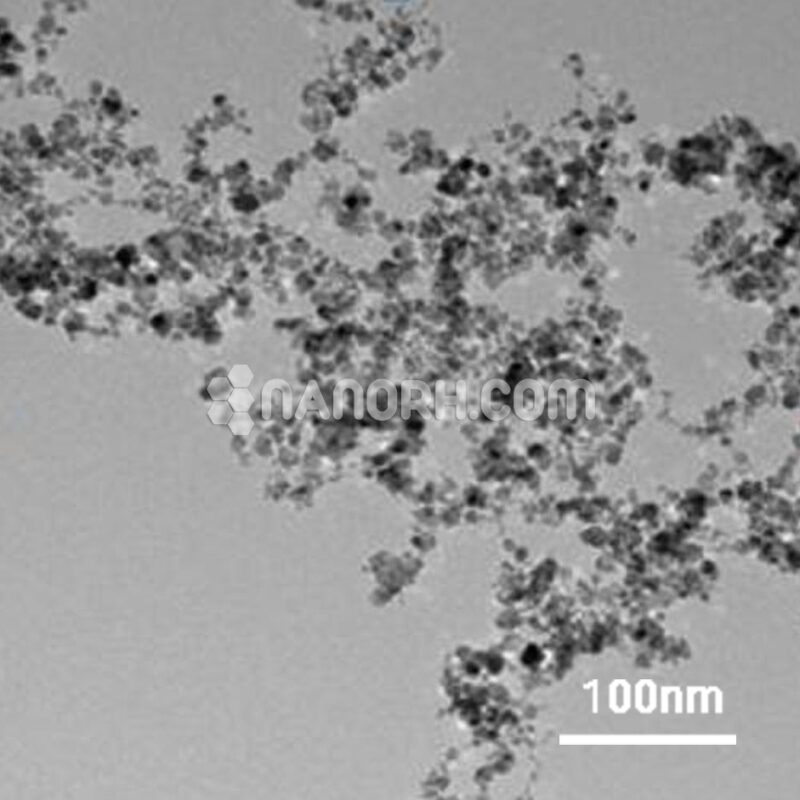
| Calcium Granules | |
| Product Number | NRE-8008 |
| CAS No. | 7440-70-2 |
| Formula | Ca |
| Molecular Weight | 40.08 g/mol |
| APS | <1-2 mm (Can be Customized) |
| Purity | 99.9% |
| Color | grey |
| Density | 1.55 g/cm3 |
| Melting Point | 842 °C |
| Boiling Point | 1484 °C |
Calcium Granules
Calcium granules, like many other forms of calcium, have various applications across different industries and fields. Calcium is an essential mineral in biology and is used in a wide range of applications due to its chemical properties and importance in human and animal health, agriculture, construction, and more. Here are some common applications of calcium granules:
Nutritional Supplements: Calcium is a vital mineral for the human body, especially for maintaining strong bones and teeth. Calcium granules can be used in the production of dietary supplements to ensure individuals meet their daily calcium requirements.
Agriculture: Calcium granules are often used in agriculture as a soil amendment to correct calcium deficiencies in soil. This can improve soil structure, reduce soil acidity, and enhance plant growth. Calcium is also applied directly to plants as a foliar spray to prevent disorders like blossom end rot in tomatoes and peppers.
Water Treatment: Calcium hydroxide (lime) is used in water treatment to raise the pH of water and reduce its acidity. It helps in the removal of impurities and metals, such as iron and manganese, from water.
Metallurgy: In metallurgical processes, calcium granules can be used as a desulfurizing agent to remove sulfur impurities from iron and steel. This helps improve the quality of the final product.
Chemical Reactions: Calcium can serve as a reactant or reagent in various chemical reactions. For example, it can be used in the production of calcium carbide, which is used in the production of acetylene gas.
Construction: Calcium is used in the construction industry as a key component of cement, concrete, and mortar. It contributes to the strength and durability of these materials.
Animal Feed: Calcium granules are used as an ingredient in animal feed to provide essential calcium for livestock and poultry. It is particularly important for eggshell formation in poultry.
Pharmaceuticals: Calcium compounds, including calcium granules, can be used in pharmaceutical formulations for various purposes, such as antacids for relieving heartburn or as a binding agent in tablet manufacturing.
Environmental Remediation: Calcium oxide (quicklime) is used in environmental remediation efforts to treat soil contaminated with heavy metals. It can immobilize or reduce the mobility of these contaminants.
Flux in Metallurgy: Calcium can act as a flux in metallurgical processes, helping to lower the melting point of various metal ores, which makes it easier to extract the desired metals.
Fireworks: Calcium is used in fireworks to produce orange-red flames. Calcium salts are often employed as colorants in pyrotechnics.
De-icing: In some areas, calcium chloride, derived from calcium, is used as a de-icing agent to melt ice on roads and sidewalks during winter.