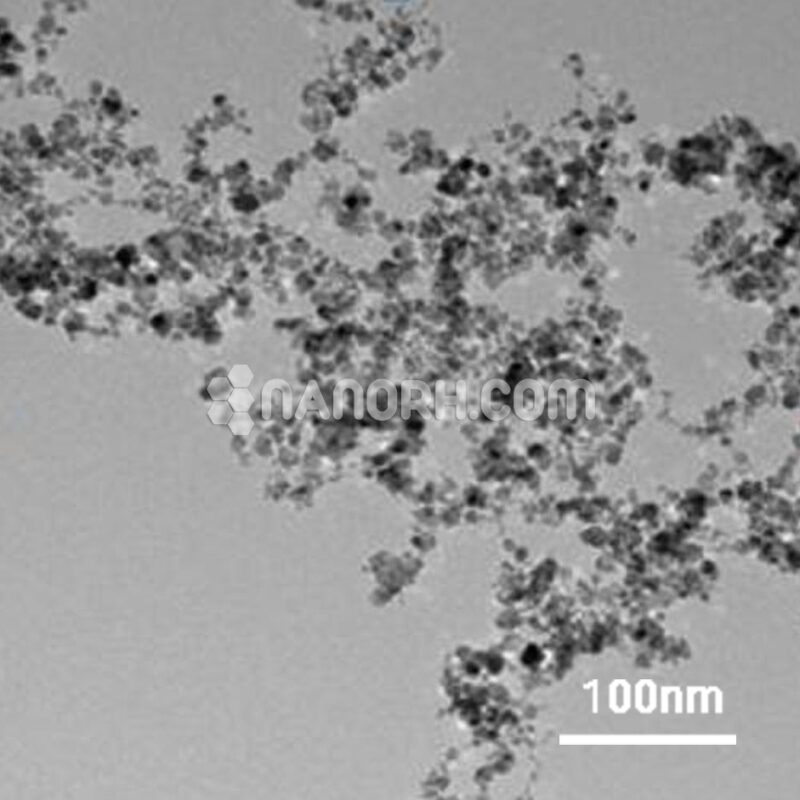
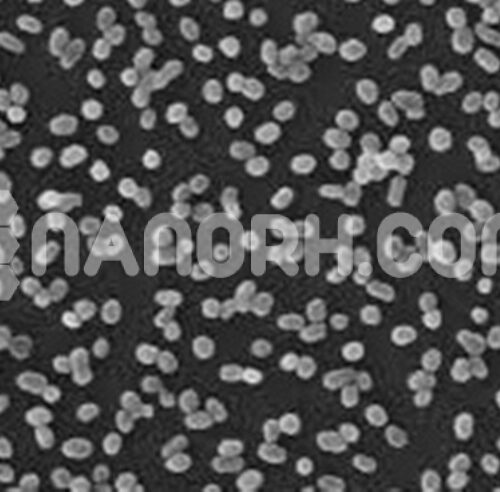
| Lithium Powder | |
| Product Number | NRE-8028 |
| CAS No. | 7439-93-2 |
| Formula | Li |
| Molecular Weight | 6.941 g/mol |
| APS | <40 µm (Can be Customized) |
| Purity | 99.9% |
| Color | White |
| Density | 0.534 g/cm3 |
| Melting Point | 180.5 °C |
| Boiling Point | 1,330 °C |
Lithium Powder
Lithium powder has several applications across various fields, owing to its unique chemical and physical properties. Here are some of the common applications of lithium powder:
Batteries: Lithium is widely used in the production of rechargeable batteries, particularly lithium-ion batteries. These batteries are commonly employed in electronic devices such as smartphones, laptops, and tablets, as well as in electric vehicles. Lithium’s high energy density and low self-discharge rate make it a preferred choice for these applications.
Ceramics and Glass: Lithium compounds are utilized in the production of ceramics and glass, where they contribute to the improvement of thermal shock resistance and reduce the melting temperature of the materials. Lithium compounds are also used as a flux in the production of glass and ceramics.
Lubricants: Lithium grease, a thickened lubricating grease containing lithium soap, is widely used in various applications, including automotive and industrial machinery. It provides excellent resistance to water and high temperatures, making it suitable for heavy-duty applications.
Pharmaceuticals: Lithium carbonate and other lithium salts are used in the treatment of certain mental health conditions, such as bipolar disorder and depression. Lithium has mood-stabilizing properties and is often prescribed as a mood-regulating medication.
Metallurgy: Lithium is used in metallurgical applications, particularly in the production of aluminum, where it acts as a degassing agent, helping to remove impurities from the metal. It is also used as an alloying agent to improve the strength and durability of metals.
Air Treatment: Lithium compounds are employed in air treatment systems, where they serve as desiccants and help in the removal of moisture from the air. This application is particularly useful in industries that require low humidity levels, such as pharmaceutical manufacturing and electronics production.
Nuclear Applications: Lithium is used in nuclear applications, including the production of tritium and as a component in the development of nuclear fusion reactors. It plays a crucial role in the advancement of nuclear energy technology.
Catalysts and Chemicals: Lithium compounds are used as catalysts in various chemical reactions, including those involved in the production of organic compounds and polymers. They also find applications in the manufacture of specialty chemicals and as additives in the production of certain plastics.