| Silicon Sulfide Nanoparticles | |
| Product No | NRE-5199 |
| CAS | 13759-10-9 |
| Purity | 99.9% |
| Formula | SiS2 |
| APS | <100 nm (Can be Customized) |
| Color | White |
| Molecular Weight | 92.22 g/mol |
| Density | 1.85 g/cm3 |
| Melting Point | 1,090° C |
| Boiling Point | NA |
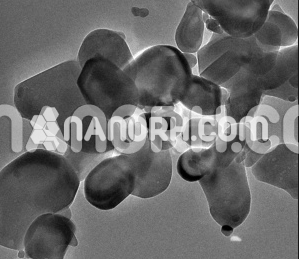
Silicon Sulfide Nanoparticles
Introduction
Silicon sulfide nanoparticles is a compound formed by the combination of silicon and sulfur. SiS2 consisting of very fine particles of this material, possess unique properties that are distinct from bulk silicon sulfide. These nanoparticles are of interest due to their exceptional reactivity, electrical conductivity, and potential for use in various high-performance applications.
Applications
Silicon sulfide nanoparticles have the potential to be used in a wide variety of applications across multiple industries, primarily due to their unique combination of electrical conductivity, high surface area, and chemical reactivity. Some of the key applications are as follows:
Energy Storage and Conversion
Lithium-ion Batteries: Silicon sulfide nanoparticles have been explored as a material for use in the anodes of lithium-ion batteries. Due to their high electrical conductivity and reactivity, they can potentially increase the energy density and charge/discharge efficiency of batteries, particularly in next-generation energy storage systems.
Supercapacitors: The high surface area and conductivity of make them an attractive candidate for use in supercapacitors, where they can help improve charge storage and enhance the performance of energy storage devices.
Solid-State Batteries: The use of silicon sulfide nanoparticles in solid-state batteries is an emerging area of research. Their ability to form solid electrolytes could lead to more stable, high-performance batteries with greater safety profiles.
Catalysis
Catalysts for Chemical Reactions: can be used as a catalyst or catalyst support in various chemical reactions, including hydrogenation, dehydrogenation, and polymerization. The high surface area of the nanoparticles allows for better catalytic efficiency, making them suitable for industrial applications in the chemical and energy sectors.
Photocatalysis: Due to their semiconductor properties, may also find applications in photocatalysis, where they can be used to accelerate chemical reactions in the presence of light. This is useful for applications such as water splitting, environmental cleanup, and energy harvesting.