| Tin Chloride | |
| Product No | NRE-5226 |
| CAS No. | 7772-99-8 |
| Formula | SnCl2 |
| APS | <100nm (Can be Customized) |
| Purity | 99.9% |
| Color | White |
| Molecular Weight | 189.60 g/mol |
| Density | 3.95 g/cm³ |
| Melting Point | 247 °C |
| Boiling Point | 623 °C |
Tin Chloride
Introduction
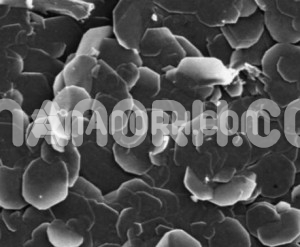
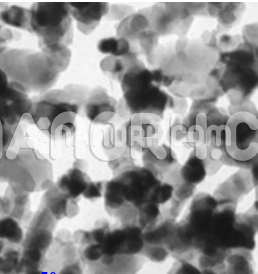
Tin chloride is an inorganic compound composed of tin (Sn) and chlorine (Cl). It exists primarily in two forms: tin(II) chloride and tin chloride (SnCl₄), with being the most common and stable form under standard conditions.
As a reducing agent and an important intermediate in various industrial processes, plays a significant role in chemistry, electronics, pharmaceuticals, and material sciences.
Key Properties:
Reducing Agent:
SnCl2 is a mild reducing agent, making it useful in reducing metal salts to their metallic forms and in other redox reactions. It is often used to reduce metallic compounds in various industrial applications.
Solubility:
Tin chloride is highly soluble in water, which allows it to participate in aqueous-based reactions and makes it suitable for use in aqueous solutions for various industrial processes.
Chemical Stability:
While is relatively stable, it can oxidize to in the presence of oxygen. This makes it sensitive to environmental conditions like air and moisture.
Non-toxic and Biocompatible:
Tin is generally considered to be non-toxic in small quantities, and is used in some biological and medical applications. However, handling precautions should still be taken in industrial settings due to its reactivity.
Catalytic Properties:
SnCl2 can be used in various catalytic processes due to its ability to facilitate oxidation and reduction reactions.