| Bismuth sulfide Powder | |
| Product No | NRE-11032 |
| CAS | 1345-07-9 |
| Purity | 99.9% |
| Formula | Bi2S3 |
| APS | <40 µm (Can be Customized) |
| Color | Brown |
| Molecular Weight | 514.16 g/mol |
| Density | 6.78 g/cm³ |
| Melting Point | 850 ˚C |
| Boiling Point | NA |
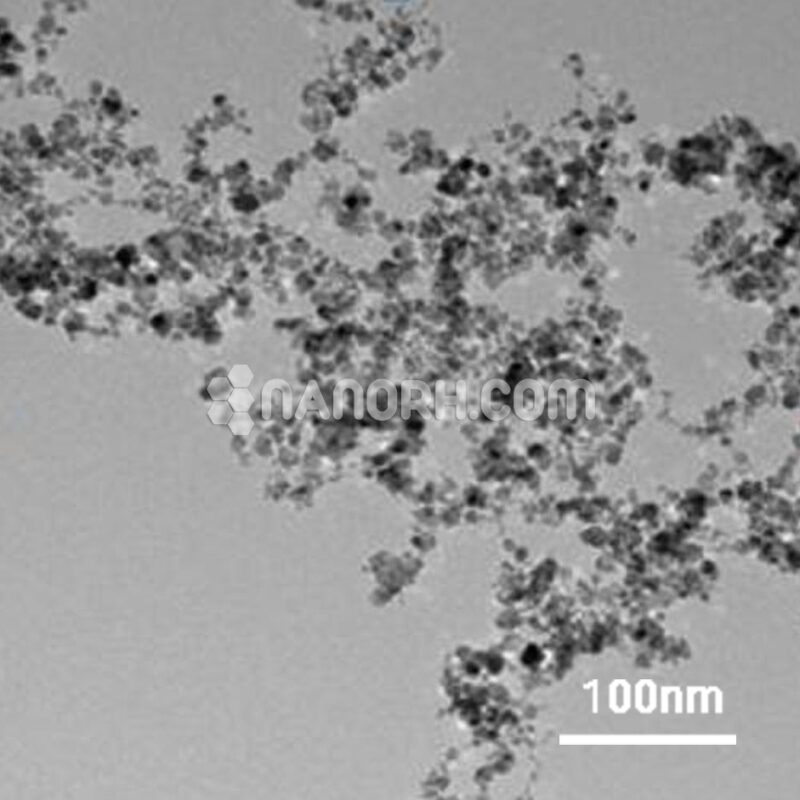
Bismuth sulfide Powder
Bismuth sulfide (Bi2S3) is a compound of bismuth and sulfur. It is commonly found in nature as the mineral bismuthinite. Bismuth sulfide powder finds various applications in different fields, owing to its unique properties. Some of the notable applications of bismuth sulfide powder include:
Photodetectors: Bismuth sulfide has been studied for its potential use in photodetectors due to its strong absorption in the visible and near-infrared spectrum. It can be used in the production of sensitive photodetectors for various applications, including light sensors and night-vision devices.
Solar Cells: Bismuth sulfide is being researched for its potential use in the development of solar cells. Its excellent light-harvesting properties and stability make it a promising material for use in photovoltaic devices. Researchers are exploring ways to enhance its efficiency and stability for commercial solar cell applications.
Thermoelectric Materials: Bismuth sulfide has been investigated as a potential thermoelectric material, capable of converting heat into electricity. Its use in thermoelectric devices is promising due to its ability to convert waste heat into usable energy, making it a potential candidate for energy harvesting applications.
Catalysts: Bismuth sulfide has shown promising catalytic activity in various reactions, such as photocatalytic water splitting and carbon dioxide reduction. Its unique properties make it a potential candidate for use as a catalyst in environmental applications and in the production of renewable energy.
Semiconductors: Bismuth sulfide is also used in the production of semiconductors, owing to its semiconducting properties. It can be employed in the development of electronic devices and integrated circuits, especially in specialized applications where its unique properties are beneficial.
Nanotechnology: Bismuth sulfide nanoparticles find applications in various fields of nanotechnology. They are used in nanoscale devices, biomedical applications, and as components in advanced materials, owing to their unique optical, electrical, and thermal properties.