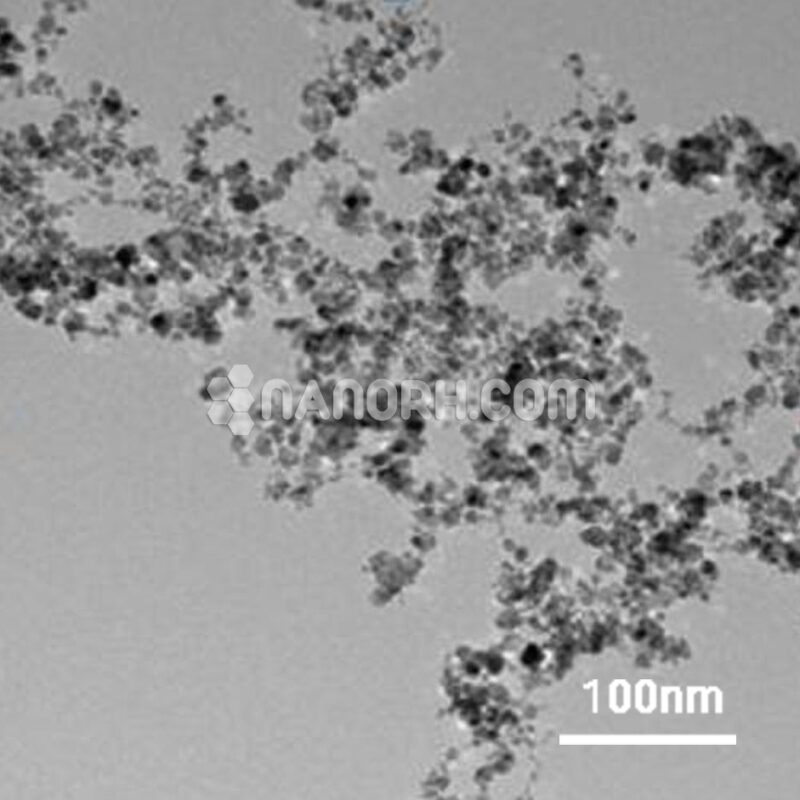
| Cobalt (Co) Powder | |
| Product No | NRE-8013 |
| CAS No. | 7440-48-4 |
| Formula | Co |
| Molecular Weight | 58.93 g/mol |
| APS | <40 um (can be customized) |
| Purity | 99.9% |
| Color | Gray Black |
| Density | 8.9 g/mL at 25 °C(lit.) |
| Melting Point | 1495 °C |
| Boiling Point | 2927 °C |
Cobalt (Co) Powder
Cobalt powder is a versatile material with a range of applications across various industries due to its unique properties. Here are some common applications of cobalt powder:
Hardfacing and Wear Resistance:
Cobalt-based powders are used in the manufacturing of hard facing materials and coatings for industrial equipment, such as mining machinery, drilling tools, and cutting tools. These coatings provide excellent wear resistance and durability.
Superalloys:
Cobalt is a key component in the production of high-temperature, corrosion-resistant superalloys used in aerospace and gas turbine engines. These alloys are known for their exceptional strength, heat resistance, and creep resistance.
Batteries:
Cobalt is a crucial component in lithium-ion batteries, which are widely used in portable electronics, electric vehicles (EVs), and renewable energy storage systems. It enhances the stability and energy density of these batteries.
Magnetic Materials:
Cobalt-based magnetic alloys are used in the manufacturing of permanent magnets for various applications, including electric motors, generators, and magnetic sensors.
Diamond Tool Production:
Cobalt powder is used in the production of diamond tools, such as cutting and grinding blades. These tools are used in industries like construction, mining, and metalworking.
Pigments and Ceramics:
Cobalt compounds are used as pigments in ceramics and glass manufacturing. Cobalt blue is a famous example of a cobalt-based pigment used in art and pottery.
Catalysts:
Cobalt-based catalysts find applications in the petrochemical industry for processes like Fischer-Tropsch synthesis and hydrogenation reactions. They are also used in catalytic converters in automobiles to reduce emissions.
Additive Manufacturing (3D Printing):
Cobalt powders are used in additive manufacturing processes like selective laser melting (SLM) and electron beam melting (EBM) to produce complex and high-performance parts for aerospace, medical, and other industries.
Medical Implants:
Cobalt-chromium alloys are used in the production of orthopedic implants, such as hip and knee replacements, due to their biocompatibility and resistance to corrosion.
Radiation Therapy:
Cobalt-60, a radioactive isotope of cobalt, is used in medical radiation therapy for cancer treatment. It emits high-energy gamma rays that can destroy cancer cells.
Alloys and Aerospace Applications:
Cobalt is used in various alloy formulations for aerospace applications, including turbine blades, aircraft engine components, and rocket nozzles.
Specialty Alloys:
Cobalt-based alloys are used in specialized applications like dental prosthetics, watch springs, and sensors.