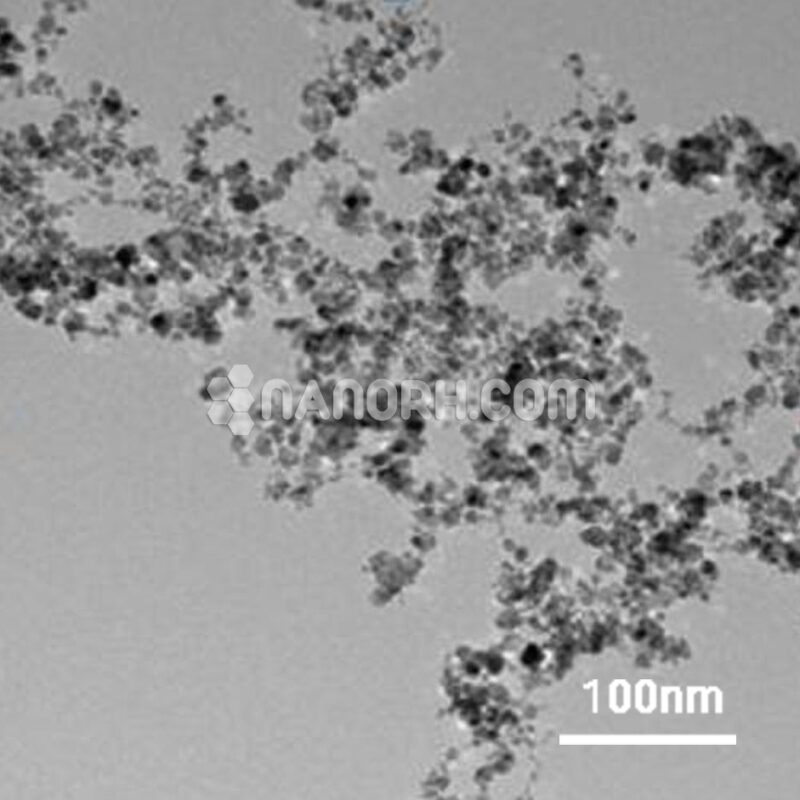
| Copper Sulfide Powder | |
| Product No | NRE-11083 |
| CAS | 22205-45-4 |
| Purity | 99.9% |
| Formula | Cu2S |
| APS | <40 µm (Can be Customized) |
| Color | NA |
| Molecular Weight | 159.16 g/mol |
| Density | 5.6 g/cm3 |
| Melting Point | 1,130 °C |
| Boiling Point | NA |
Copper Sulfide Powder
Copper sulfide powder, composed of copper and sulfur, finds applications in various fields due to its unique properties. Some of its common applications include:
Catalysts: Copper sulfide powder is often used as a catalyst in various chemical reactions, including hydrogenation and dehydrogenation processes.
Semiconductors: Copper sulfide is utilized in the production of semiconductors due to its semiconducting properties. It is employed in the manufacture of photodetectors, solar cells, and thermoelectric devices.
Lubricants: Copper sulfide powder can be added to lubricants to improve their anti-wear and friction-reducing properties. It helps reduce friction and wear between moving parts, thereby extending the lifespan of machinery and equipment.
Pigments: In the field of art and industry, copper sulfide can be used as a pigment in the production of various shades of black and grey colors.
Batteries: Copper sulfide is being explored for its potential application in lithium-ion batteries and other energy storage devices. Its conductivity and stability make it a promising candidate for the development of high-performance batteries.
Photovoltaic Devices: Copper sulfide is used in the production of solar cells and other photovoltaic devices due to its ability to absorb sunlight and convert it into electricity.
Thermoelectric Materials: Copper sulfide can be used in thermoelectric materials to convert heat energy into electrical energy. It is being researched for its potential application in waste heat recovery systems.
Infrared Detectors: Copper sulfide has the property of detecting infrared radiation, making it suitable for use in infrared detectors and thermal imaging devices.
Anti-microbial Agents: Copper sulfide nanoparticles have been investigated for their antimicrobial properties, and they are being explored for potential applications in various antimicrobial products and treatments.