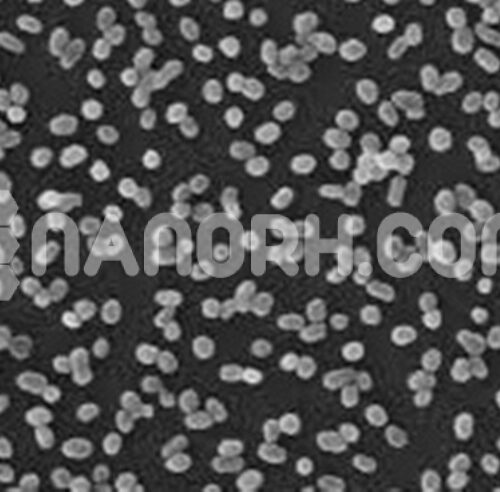
| Iron Powder / Fe Powder | |
| Product No | NRE-8026 |
| CAS No. | 7439-89-6 |
| Formula | Fe |
| Molecular Weight | 55.845 g/mol |
| APS | 800nm( can be customized) |
| Purity | 99.9% metal basis |
| Color | dark grey |
| Density | 7.874 g/cm³ |
| Melting Point | 1538 °C |
| Boiling Point | 2862 °C |
Iron Powder / Fe Powder
Iron powder has a wide range of applications in various industries due to its unique properties. Some of the common applications of iron powder include:
Metallurgy and Steel Production: Iron powder is a crucial raw material for the production of steel and other ferrous alloys. It is often used as a core material in the production of sintered components.
Automotive Industry: Iron powder is used to make sintered components for automobiles, such as gears, bearings, and bushings. These components offer high strength and wear resistance.
Manufacturing and Machinery: Iron powder is used in the production of various machine parts and tools. It can be mixed with other materials to create high-strength, wear-resistant components.
Electromagnetic Applications: Iron powder is used in the manufacture of inductors and electromagnetic components, where its magnetic properties are essential.
Chemical Industry: Iron powder is used as a reducing agent in chemical processes. It is also used in the production of various chemicals and catalysts.
Welding and Brazing: Iron powder is often used in welding and brazing applications to improve the flow of molten metal and to create stronger joints.
Additive Manufacturing (3D Printing): Iron powder can be used in powder metallurgy-based 3D printing technologies to create complex and strong metal parts.
Agriculture: Iron powder is used in agricultural applications, especially in fertilizers, to provide essential micronutrients to plants.
Friction Materials: Iron powder is incorporated into brake pads and clutch plates to enhance their wear resistance and heat dissipation properties.
Magnetic Materials: Iron powder can be used to create magnetic materials for various applications, including magnetic recording media, sensors, and magnetic shielding.
Surface Coatings: Iron powder can be used in surface coatings to improve the wear resistance and corrosion resistance of various products, including machinery and appliances.
Environmental Remediation: In some cases, iron powder is used in environmental remediation processes to remove contaminants from soil and groundwater through chemical reactions.
Fireworks: Iron powder is used as a pyrotechnic ingredient in fireworks to produce bright and sparkling effects.
Art and Craft: Iron powder can be used for artistic and craft applications, creating unique textures and effects in various artistic projects.
Medicine: In the medical field, iron powder can be used in certain diagnostic tests and as a contrast agent in medical imaging.